Efarindodekin alfa, a tumor-activated IL-12
Xilio is focused on developing cytokines with exemplary clinical activity and tolerability. We are developing efarindodekin alfa, a tumor-activated IL-12, for the treatment of advanced solid tumors.
About IL-12
IL-12, or Interleukin-12, has emerged as one of the most potent cytokine mediators of antitumor activity because of its multiple effects on different immune cells in the tumor microenvironment (TME). IL-12 has the ability to activate and bridge both innate (natural killer or NK cell) and adaptive (cytotoxic CD8+ T and CD4+ T cells) immunity for tumor cell killing while further coordinating additional anti-cancer defenses such as regulatory T-cell suppression and anti-angiogenic effects. Despite research across the field suggesting that IL-12 can significantly benefit patients in fighting cancer, there are currently no approved IL-12 agents due to severe systemic toxicity with unmodified IL-12.
Efarindodekin Alfa, a Tumor-activated IL-12
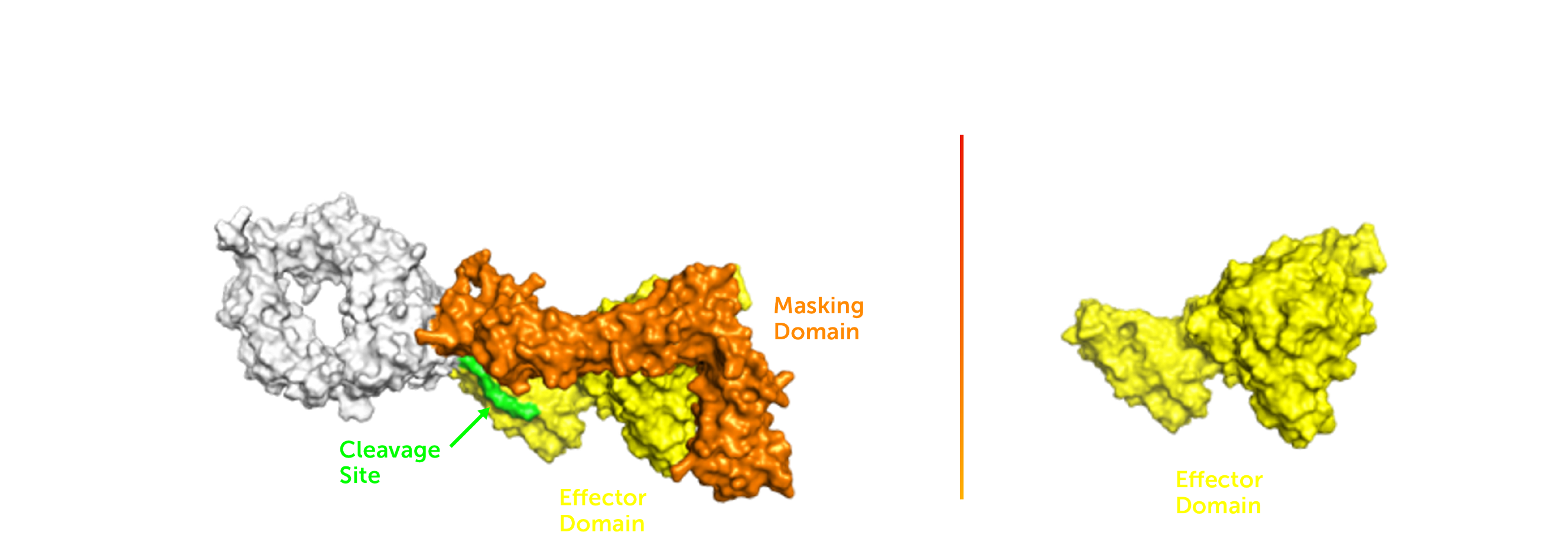
Efarindodekin alfa is an investigational tumor-activated IL-12 molecule designed to potently stimulate anti-tumor immunity and reprogram the TME of poorly immunogenic “cold” tumors towards an inflamed or “hot” state.
Efarindodekin alfa is currently being evaluated in an ongoing Phase 1/2 clinical trial as a monotherapy in patients with advanced solid tumors. Significant potential also exists to explore the development of efarindodekin alfa in combination with other agents and modalities.
About the Phase 1/2 Trial for Efarindodekin Alfa
Efarindodekin alfa is an investigational masked IL-12 designed to potently stimulate anti-tumor immunity and reprogram the tumor microenvironment (TME) of poorly immunogenic “cold” tumors towards an inflamed or “hot” state. In March 2024, Xilio entered into an exclusive license agreement with Gilead Sciences, Inc. for Xilio’s tumor-activated IL-12 program, including efarindodekin alfa. Xilio is currently evaluating the safety and tolerability of efarindodekin alfa as a monotherapy in patients with advanced solid tumors in the Phase 1 portion of a first-in-human, multi-center, open-label Phase 1/2 clinical trial and the safety and efficacy of efarindodekin alfa as a monotherapy in the Phase 2 portion in patients with advanced solid tumors. The Phase 2 portion of the trial is anticipated to enroll approximately 40 patients in specific tumor types at multiple sites in the United States. Please refer to NCT05684965 on www.clinicaltrials.gov for additional details.
Efarindodekin Alfa (XTX301) Presentations & Publications
11/07/2025
Efarindodekin alfa (XTX301) Poster Presentation at the 2025 Society for Immunotherapy of Cancer (SITC) Annual Meeting
XTX301, a Tumor-Activated Interleukin-12 (IL-12), Demonstrated IL-1 Pharmacology in Patients with Advanced Solid Tumors: Pharmacodynamic Data from First-in-Human Phase 1 Study (NCT05684965)
11/30/2023
XTX301 Publication in Molecular Cancer Therapeutics
XTX301, a tumor-activated Interleukin-12 has the potential to widen the therapeutic index of IL-12 treatment for solid tumors as evidenced by pre-clinical studies
11/04/2023
XTX301 Poster Presentation at the 2023 Society for Immunotherapy of Cancer’s (SITC) 38th Annual Meeting
XTX301, a tumor-activated, half-life extended IL-12 promoted potent anti-tumor immunity and activity across multiple syngeneic tumor models
06/30/2023
XTX301 Poster Presentation at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting
A first-in-human study of XTX301, a masked, tumor-activated interleukin-12 (IL-12) in patients with advanced solid tumors
More XILIO News
04/16/2023
XTX301 Poster Presentation at the American Association for Cancer Research (AACR) 2023 Annual Meeting
A half-life extended, tumor-activated IL-12 increased the infiltration of effector immune cells into the tumor microenvironment and demonstrated anti-tumor activity in syngeneic mouse models
05/10/2022
XTX301 Poster Presentation at the New York Academy of Sciences’ Frontiers in Cancer Immunotherapy 2022 Conference
A half-life extended, tumor selective IL-12 activated tumor infiltrating immune cells and demonstrated anti-tumor activity in the MC38 syngeneic mouse model
11/12/2021
XTX301 Poster Presentation at the Society for Immunotherapy in Cancer’s 36th Annual Meeting (SITC)
XTX301, A Protein-engineered IL-12, Exhibits Tumor-selective Activity In Mice Without Peripheral Toxicities And Is Well Tolerated In Non-Human Primates