XTX202, a tumor-activated, beta-gamma biased, engineered IL-2
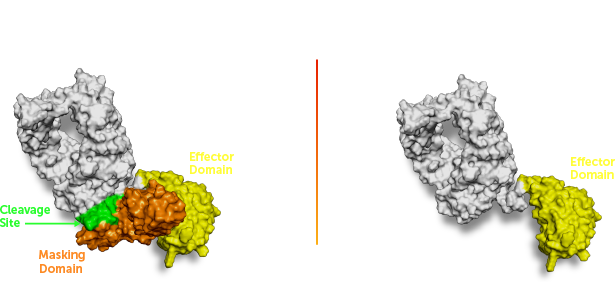
XTX202 is an investigational tumor-activated, beta-gamma biased, engineered IL-2 molecule designed to potently stimulate CD8+ effector T cells and natural killer (NK) cells without concomitant stimulation of regulatory T cells when activated (unmasked) in the tumor microenvironment.
About IL-2
IL-2, or Interleukin-2, is a type of cytokine that regulates the activities of white blood cells by binding to IL-2 receptors. IL-2 is currently used as a biological response modifier to boost the immune system in cancer therapy. Drug development of and treatment with IL-2 therapies has been limited due to severe systemic toxicity, including fatal outcomes.
About the Phase 1/2 Clinical Trial for XTX202
The Phase 1 clinical trial for XTX202 is a first-in-human, multi-center, open-label trial designed to evaluate the safety and tolerability of XTX202 as a monotherapy in patients with advanced solid tumors. The Phase 2 clinical trial for XTX202 is a multi-center, open-label trial designed to evaluate the safety and efficacy of XTX202 as a monotherapy in patients with unresectable or metastatic melanoma and metastatic renal cell carcinoma who have progressed on standard-of-care treatment. Enrollment has been completed in the Phase 1 and Phase 2 clinical trials. Please refer to NCT05052268 on www.clinicaltrials.gov for additional details.
XTX202 Presentations and Publications
06/01/2024
XTX202 Poster Presentation at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting
Phase 1/2 Study of XTX202, a Tumor-Activated IL-2βγ in Advanced Solid Tumors
11/03/2023
XTX202 Poster Presentation at the 2023 Society for Immunotherapy of Cancer’s (SITC) 38th Annual Meeting
XTX202-01, Phase 1/2 first-in-human study of XTX202, a masked, tumor-activated IL-2βγ, in patients with advanced solid tumors
11/10/2022
XTX202 Poster Presentation at the 2022 Society for Immunotherapy of Cancer’s (SITC) 37th Annual Meeting
XTX202, a tumor-selective protein-engineered IL-2, exhibited enhanced anti-tumor activity in combination with checkpoint inhibition in mice
06/05/2022
XTX202 Poster Presentation at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting
A First-in-Human, Multicenter, Phase 1/2, Open-Label Study of XTX202, a Tumor-Selective Interleukin-2 (IL-2), in Patients with Advanced Solid Tumors
More XILIO News
06/08/2021
XTX202 (IL-2) Poster Presentation at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting
XTX202, a protein-engineered IL-2, exhibits tumor-selective activity in mice without peripheral toxicities in non-human primates
11/12/2020
XTX201 (IL-2) Poster Presentation at 35th Annual Meeting of The Society for Immunotherapy of Cancer
XTX201, a protein-engineered IL-2, exhibits tumor-selective activity in mice without peripheral toxicities in non-human primates